
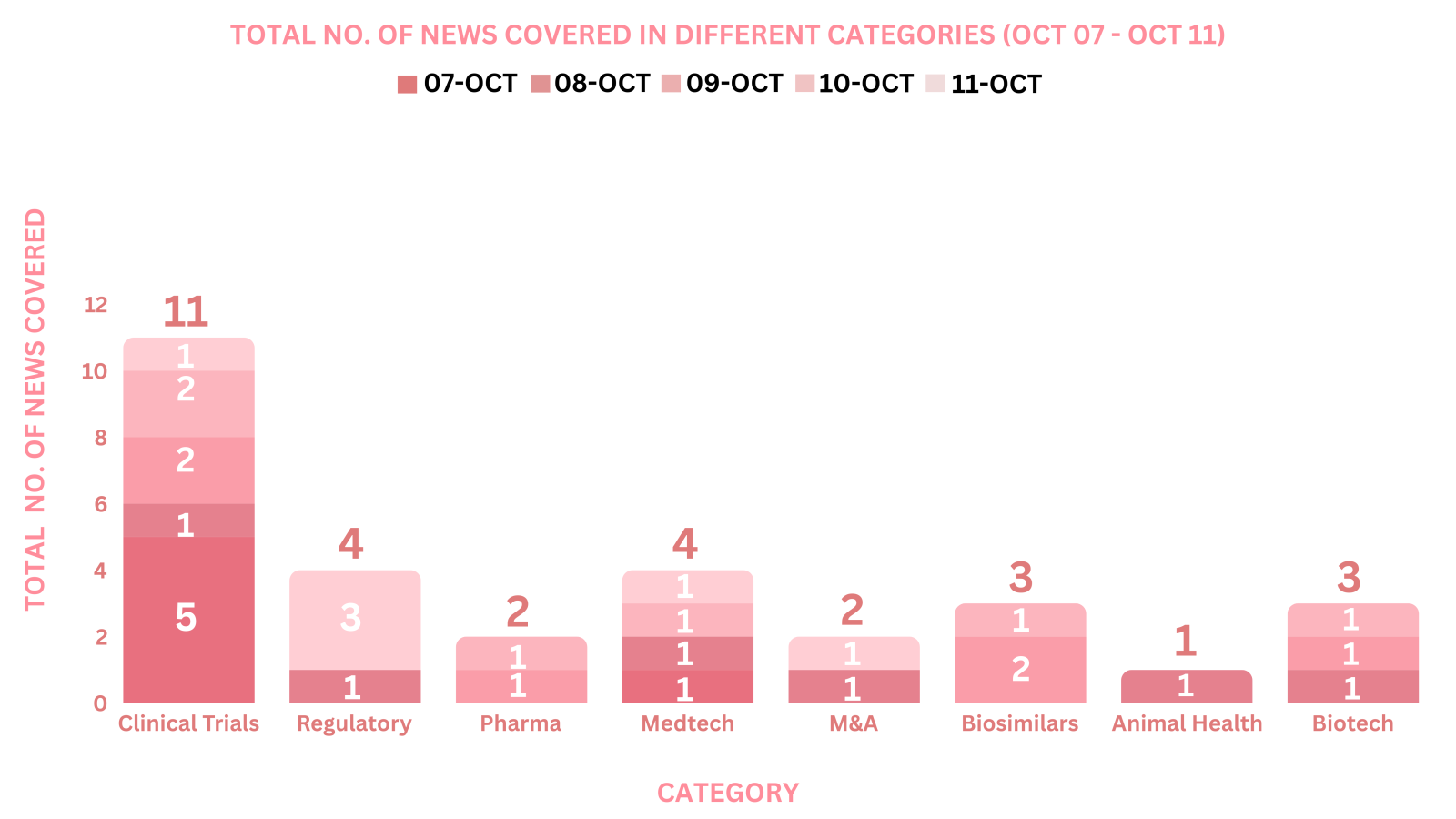
PharmaShots Weekly Snapshots (October 07 – October 11, 2024)
This week PharmaShots’ news was all about the updates on Clinical Trials, Pharma, Biotech, M&A, Biosimilar, Animal Health, Regulatory & MedTech. Check out our full report below:

AstraZeneca Reports the Data from P-IIIb (BATURA) Study of Airsupra (Albuterol/Budesonide) for Intermittent or Mild Persistent Asthma
Read More: AstraZeneca
GE HealthCare Reports the P-I Study Data of its Macrocyclic Manganese-Based MRI Contrast Agent
Read More: GE HealthCare
Hansa Biopharma Reports the Results from the NICE-01 Clinical Evaluation of HNSA-5487
Read More: Hansa Biopharma
BerGenBio Reports the Data from P-Ib/IIa (BGBC016) Clinical Evaluation of Bemcentinib as 1L Treatment of NSCLC
Read More: BerGenBio
Cybrexa Therapeutics Doses the First Patient with CBX-12 Under P-II Study for Treating Ovarian Cancer
Read More: Cybrexa Therapeutics
Sage Therapeutics Provides Data from the P-II (LIGHTWAVE) Trial of Dalzanemdor to Treat Mild Cognitive Impairment and Dementia in Alzheimer’s Disease
Read More: Sage Therapeutics
Merck Provides Update on P-III (KEYNOTE-689) Study of Keytruda as a Perioperative Treatment of Head and Neck Squamous Cell Carcinoma
Read More: Merck
Aurigene Oncology Reports Results from the P-I (SWASTH) Trial of Ribrecabtagene Autoleucel for Multiple Myeloma in India
Read More: Aurigene Oncology
Oncoinvent Reports the First Patient Dosing with Radspherin in its P-II Clinical Evaluation for Ovarian Cancer
Read More: Oncoinvent
Pfizer Reports the Data from P-III (TALAPRO-2) Study of Talzenna Plus Xtandi for Treating mCRPC
Read More: Pfizer
Johnson & Johnson Reports Tremfya Pooled Analysis from GALAXI-2, 3 and QUASAR Trials
Read More: Johnson & Johnson

Zealand Pharma and Boehringer Ingelheim Report the US FDA’s Breakthrough Therapy Designation of Survodutide for MASH
Read More: Zealand Pharma and Boehringer Ingelheim
Biogen’s Felzartamab Gains the US FDA’s Breakthrough Therapy Designation to Treat AMR in Kidney Transplant Recipients
Read More: Biogen
Roche’s Reports the US FDA’s Approval of Itovebi as a Treatment for HR+ and HER2- breast cancer with a PIK3CA mutation
Read More: Roche
Burning Rock and Dizal Reports China's NMPA Approval of Co-Developed Companion Diagnostic (CDx) for Lung Cancer
Read More: Burning Rock and Dizal

Astellas Join Forces with AviadoBio for AVB-101 Gene Therapy Targeting Frontotemporal Dementia and Other Indications
Read More: Astellas and AviadoBio
Boehringer Ingelheim Join Forces with Circle Pharma to Develop a Novel Precision Cancer Treatment
Read More: Boehringer Ingelheim and Circle Pharma

Exact Sciences Reports the US FDA’s Approval of Cologuard Plus Test for Non-Invasive Colorectal Cancer Screening
Read More: Exact Sciences
The US FDA Grants Approval to ALK’s Series of AccuTest Devices for Allergy Testing and Diagnosis
Read More: ALK
Roche’s Ventana CLDN18 (43-14A) RxDx Assay Receives CE Mark Approval to Diagnose G/GEJ Cancer Patients with CLDN18 Protein Expression
Read More: Roche
RIVANNA launches a Global FIH Trial for its Accuro XV Musculoskeletal Imaging System
Read More: RIVANNA

Caldera Medical Reports the Acquisition of UVision360, Strengthening its Minimally Invasive Hysteroscopy Portfolio
Read More: Caldera Medical
Johnson & Johnson Completes the Acquisition of V-Wave
Read More: Johnson & Johnson and V-Wave

Teva Reports the Acceptance of Regulatory Submissions for TVB-009P (Biosimilar, Prolia) by the US FDA and EMA
Read More: Teva Pharmaceuticals
Bio-Thera Collaborates with Gedeon Richter for BAT2206 (Biosimilar, Stelara)
Read More: Bio-Thera and Gedeon Richter
Alvotech Reports the EMA’s Acceptance of MAA for AVT03 (Biosimilar, Prolia and Xgeva)
Read More: Alvotech

Elanco Animal Health Reports the US FDA’s Approval of Credelio Quattro for Protection Against Parasites in Dogs
Read More: Elanco Animal Health

AstraZeneca Inks a Pact with CSPC Pharmaceutical Group to Advance a Lipid-Lowering Pre-Clinical Candidate
Read More: AstraZeneca and CSPC Pharmaceutical
Bayer Collaborates with MOMA Therapeutics for the Development of a Small Molecule Oncology Program
Read More: Bayer and MOMA Therapeutics
Revalesio Reports Results from its Non-Clinical Study of RNS60 for Amyotrophic Lateral Sclerosis (ALS)
Read More: Revalesio
Related Post: PharmaShots Weekly Snapshots (September 30 – October 04, 2024)
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














